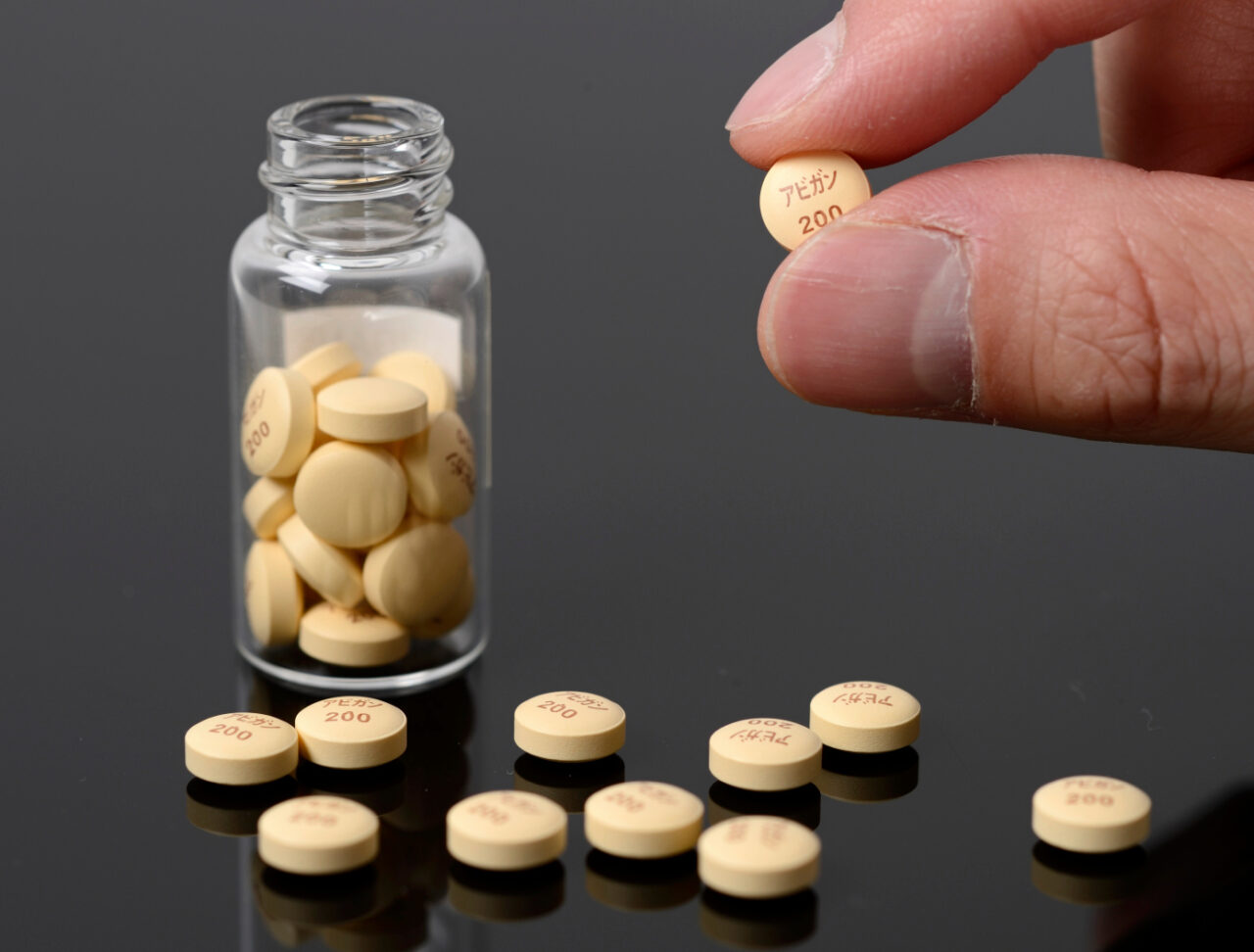
Avigan influenza tablets, produced by Fujifilm Holdings Corp., are arranged for a photograph at the company’s headquarters in Tokyo, Japan, on Monday, Oct. 27, 2014. Fujifilm, which makes the Avigan drug used as part of clinical trials in Ebola patients, said aid group Doctors Without Borders may use the drug among its experimental treatments. Photographer: Akio Kon/Bloomberg via Getty Images
Avigan influenza tablets, produced by Fujifilm Holdings Corp., are arranged for a photograph at the company’s headquarters in Tokyo, Japan, on Monday, Oct. 27, 2014. Fujifilm, which makes the Avigan drug used as part of clinical trials in Ebola patients, said aid group Doctors Without Borders may use the drug among its experimental treatments. Photographer: Akio Kon/Bloomberg via Getty ImagesTablets of Avigan, developed and manufactured by Japanese drug maker Toyama Chemical Co., Ltd. , are seen at Fujifilm Holdings headquarters in Tokyo, Japan, on Monday, Oct. 27, 2014. Photographer: Akio Kon/ BloombergBased on results of clinical trials conducted with affected patients in both Wuhan and Shenzhen by Chinese medical authorities, Japanese-made flu drug favipiravir (also known as Avigan) has been shown to be effective in both reducing the duration of the COVID-19 virus in patients and to have improved the lung conditions of those who received treatment with the drug.